Running FASTQC to Assess Read Quality
Overview
Questions
- How can I describe the quality of my data?
Objectives
Explain how a FASTQ file encodes per-base quality scores.
Interpret a FastQC plot summarizing per-base quality across all reads.
Use
forloops to automate operations on multiple files.How to download data from the HPC
Bioinformatic workflows
When working with high-throughput sequencing data, the raw reads you get off of the sequencer will need to pass through a number of different tools in order to generate your final desired output. The execution of this set of tools in a specified order is commonly referred to as a workflow or a pipeline.
An example of the RNA workflow is provided below.
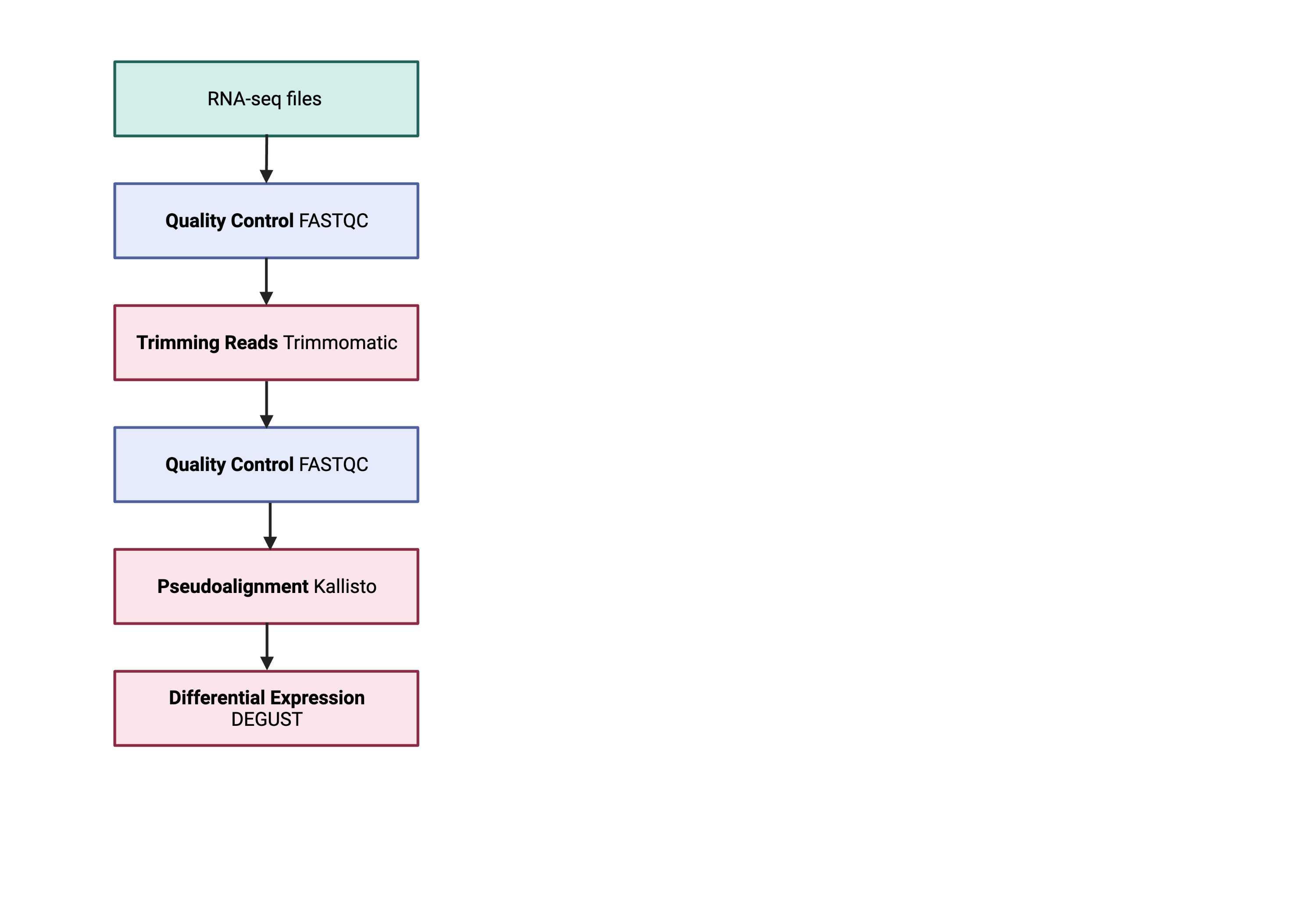
- Quality control - Assessing quality using FastQC
- Quality control - Trimming and/or filtering reads (if necessary)
- Align reads to reference genome
- Differential expression DESeq2
Standard data formats are essential and used throughout bioinformatics. We will be only performing quality control to check the quality of FastQC.
Starting with data
Recap
What is the difference between single and paired-end reads?
With paired-end sequencing, both ends of the fragment are sequenced. With single-end sequencing, only one end of a fragment is sequenced. If the data is paired-end, you have two files for each sample.
To download the data, please:
1) Request an interactive session using qsub
2) Use mkdir to create a folder for your input fastq file e.g. data
You can use the -p option for mkdir. This option allows mkdir to create the new directory, even if one of the parent directories does not already exist. It also suppresses errors if the directory already exists, without overwriting that directory.
3) Download the dataset below to your local scratch
$ mkdir -p /scratch/im21/[your_userid]/fastq_data/
$ cd /scratch/im21/[your_userid]/fastq_data/
$ wget ftp://ftp.sra.ebi.ac.uk/vol1/fastq/SRR258/004/SRR2589044/SRR2589044_1.fastq.gz
$ wget ftp://ftp.sra.ebi.ac.uk/vol1/fastq/SRR258/004/SRR2589044/SRR2589044_2.fastq.gz
The data comes in a compressed format, so there is a .gz at the end of the file names. This makes it faster to transfer and allows it to take up less space on our computer. To inspect the contents of a FASTQ file, you can decompress it and view it with tools like less or zcat.
$ gunzip SRR2589044_1.fastq.gz #do not do this!!!
Quality control
We will now assess the quality of the sequence reads contained in our fastq files.
We can view the first complete read in one of the files our dataset by using head to look at the first four lines.
$ zcat SRR2589044_1.fastq.gz | head -n 4
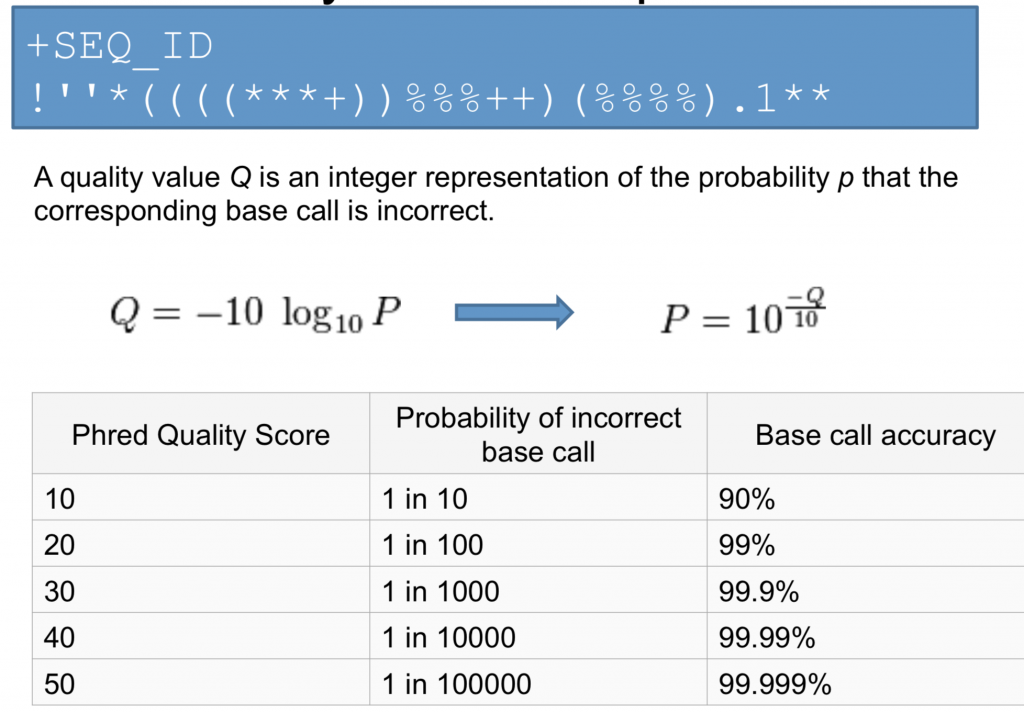
Line 4 shows the quality for each nucleotide in the read. Quality is interpreted as the probability of an incorrect base call (e.g. 1 in 10) or, equivalently, the base call accuracy (e.g. 90%). To make it possible to line up each nucleotide with its quality score, the numerical score is converted into a code where each character represents the numerical quality score for an individual nucleotide. For example, in the line above, the quality score line is:
!69699><;;:8=+:::::987765979858859775775883796699+48789599878592274362843111
The numerical value assigned to each character depends on the sequencing platform that generated the reads. The sequencing machine to generate our data uses the standard Sanger quality PHRED score encoding, using Illumina version 1.8 onwards. Each character is assigned a quality score between 0 and 41 as shown in the chart below.
Quality encoding: !"#$%&'()*+,-./0123456789:;<=>@ABCDEFGHI
| | | | |
Quality score: 01........11........20........30.......40
Each quality score represents the probability that the corresponding nucleotide call is incorrect. This quality score is logarithmically based, so a quality score of 10 reflects a base call accuracy of 90%, but a quality score of 20 reflects a base call accuracy of 99%. These probability values are the results from the base calling algorithm and depend on how much signal was captured for the base incorporation.
Looking back at our read:
@SRR306844.16.1
NTGTAAATGAGTGAGGCAGGAGTCCGAGGAGGTTAGTTGTGGCAATAAAAATGATTAAGGATACTAGTATAAGAGA
+
!69699><;;:8=+:::::987765979858859775775883796699+48789599878592274362843111
We can now see that there is a range of quality scores, but that the end of the sequence is very poor (! = a quality score of 1).
Exercise
What is the last read in your file? Do you know if this is of high quality? Explain. Hint: Use the command: tail
Loading a new function
Try to run fastqc to see if it is available; if not, we will “load” it into your local path.
$ fastqc
You will not be asked to install any packages in this course. The functions will be available through a main installation but you will need to modify your local environment so your computer knows exactly where the main installation is.
$ module avail
To find modules of interest, you can use the grep command to filter out a string. E.g. any package with “t”
$ module avail | grep fas
Exercise
Which function would you use to filter the output of module avail to show only output with keyword “fastq” ?
To load the function. You need to first load the path where fastqc is located.
$ module load fastqc/0.11.8
Please run the command below to output the command line options.
$ fastqc -h
FastQC - A high throughput sequence QC analysis tool
SYNOPSIS
fastqc seqfile1 seqfile2 .. seqfileN
fastqc [-o output dir] [--(no)extract] [-f fastq|bam|sam]
[-c contaminant file] seqfile1 .. seqfileN
DESCRIPTION
FastQC reads a set of sequence files and produces from each one a quality
control report consisting of a number of different modules, each one of
which will help to identify a different potential type of problem in your
data.
If no files to process are specified on the command line then the program
will start as an interactive graphical application. If files are provided
on the command line then the program will run with no user interaction
required. In this mode it is suitable for inclusion into a standardised
analysis pipeline.
The options for the program as as follows:
-h --help Print this help file and exit
-v --version Print the version of the program and exit
-o --outdir Create all output files in the specified output directory.
Please note that this directory must exist as the program
will not create it. If this option is not set then the
output file for each sequence file is created in the same
directory as the sequence file which was processed.
--casava Files come from raw casava output. Files in the same sample
group (differing only by the group number) will be analysed
as a set rather than individually. Sequences with the filter
flag set in the header will be excluded from the analysis.
Files must have the same names given to them by casava
(including being gzipped and ending with .gz) otherwise they
will not be grouped together correctly.
--nano Files come from naopore sequences and are in fast5 format. In
this mode you can pass in directories to process and the program
will take in all fast5 files within those directories and produce
a single output file from the sequences found in all files.
--nofilter If running with --casava then don't remove read flagged by
casava as poor quality when performing the QC analysis.
--extract If set then the zipped output file will be uncompressed in
the same directory after it has been created. By default
this option will be set if fastqc is run in non-interactive
mode.
-j --java Provides the full path to the java binary you want to use to
launch fastqc. If not supplied then java is assumed to be in
your path.
--noextract Do not uncompress the output file after creating it. You
should set this option if you do not wish to uncompress
the output when running in non-interactive mode.
--nogroup Disable grouping of bases for reads >50bp. All reports will
show data for every base in the read. WARNING: Using this
option will cause fastqc to crash and burn if you use it on
really long reads, and your plots may end up a ridiculous size.
You have been warned!
-f --format Bypasses the normal sequence file format detection and
forces the program to use the specified format. Valid
formats are bam,sam,bam_mapped,sam_mapped and fastq
-t --threads Specifies the number of files which can be processed
simultaneously. Each thread will be allocated 250MB of
memory so you shouldn't run more threads than your
available memory will cope with, and not more than
6 threads on a 32 bit machine
-c Specifies a non-default file which contains the list of
--contaminants contaminants to screen overrepresented sequences against.
The file must contain sets of named contaminants in the
form name[tab]sequence. Lines prefixed with a hash will
be ignored.
-a Specifies a non-default file which contains the list of
--adapters adapter sequences which will be explicity searched against
the library. The file must contain sets of named adapters
in the form name[tab]sequence. Lines prefixed with a hash
will be ignored.
-l Specifies a non-default file which contains a set of criteria
--limits which will be used to determine the warn/error limits for the
various modules. This file can also be used to selectively
remove some modules from the output all together. The format
needs to mirror the default limits.txt file found in the
Configuration folder.
-k --kmers Specifies the length of Kmer to look for in the Kmer content
module. Specified Kmer length must be between 2 and 10. Default
length is 7 if not specified.
-q --quiet Supress all progress messages on stdout and only report errors.
-d --dir Selects a directory to be used for temporary files written when
generating report images. Defaults to system temp directory if
not specified.
BUGS
Any bugs in fastqc should be reported either to [email protected]
or in www.bioinformatics.babraham.ac.uk/bugzilla/
Assessing quality using FastQC
In real life, you will not be assessing the quality of your reads by visually inspecting your FASTQ files. Rather, you will be using a software program to assess read quality and filter out poor-quality reads. We will first use a program called FastQC to visualise the quality of our reads. Later in our workflow, we will use another program to filter out poor-quality reads.
FastQC has several features that can give insight into any problems your data may have. This can stop you from carrying issues forward in your analyses. Rather than looking at quality scores for each individual read, FastQC looks at quality collectively across all reads within a sample. The image below shows one FastQC-generated plot that indicates a very high-quality sample:
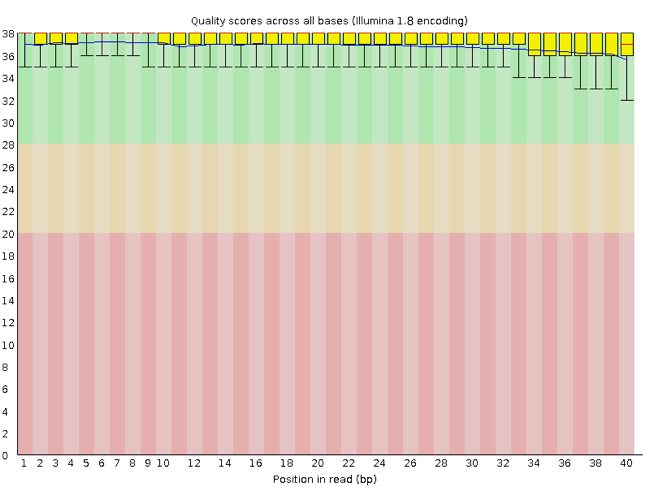
The x-axis displays the base position in the read, and the y-axis shows quality scores. In this example, the sample contains reads that are 40 bp long. This is much shorter than the reads we are working with in our workflow. For each position, there is a box-and-whisker plot showing the distribution of quality scores for all reads at that position. The horizontal red line indicates the median quality score and the yellow box shows the 1st to 3rd quartile range. This means that 50% of reads have a quality score that falls within the range of the yellow box at that position. The whiskers show the absolute range, which covers the lowest (0th quartile) to the highest (4th quartile) values.
For each position in this sample, the quality values do not drop much lower than 32. This is a high-quality score. The plot background is also colour-coded to identify good (green), acceptable (yellow), and bad (red) quality scores.
Now let’s look at a quality plot on the other end of the spectrum.
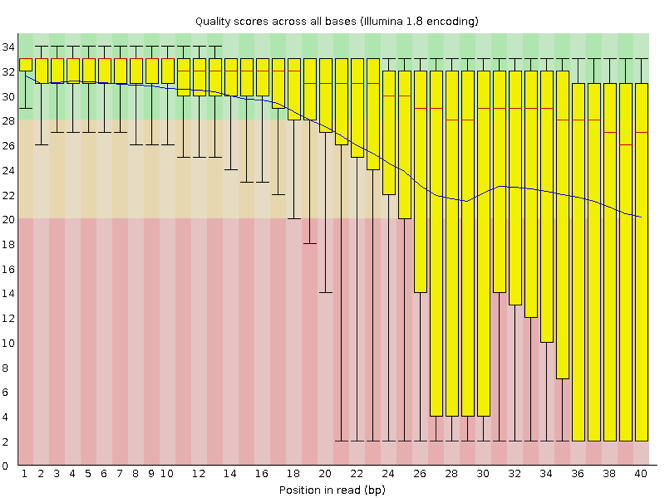
Here, we see positions within the read in which the boxes span a much wider range. Also, quality scores drop quite low into the “bad” range, particularly on the tail end of the reads. The FastQC tool produces several other diagnostic plots to assess sample quality, in addition to the one plotted above.
Running FastQC
We will now assess the quality of the reads that we downloaded. First, make sure you are still in the fastq_data directory.
$ cd /scratch/im21/[your_userid]/fastq_data/
Exercise
How big are your files? (Hint: Look at the options for the
lscommand to see how to show file sizes.)
FastQC can accept multiple file names as input, and on both zipped and unzipped files, so we can use the *.fastq* wildcard to run FastQC on all of the FASTQ files in this directory.
$ fastqc *.fastq*
You will see an automatically updating output message telling you the progress of the analysis. It will start like this:
Started analysis of SRR2589044_1.fastq.gz
Approx 5% complete for SRR2589044_1.fastq.gz
Approx 10% complete for SRR2589044_1.fastq.gz
Approx 15% complete for SRR2589044_1.fastq.gz
The FastQC program has created several new files within our /data/ directory.
$ ls
For each input FASTQ file, FastQC has created a .zip file and a
.html file. The .zip file extension indicates that this is actually a compressed set of multiple output files. We will be working with these output files soon. The .html file is a stable webpage displaying the summary report for each of our samples.
We want to keep our data files and our results files separate, so we will move these output files into a new directory within our results/ directory.
$ mkdir -p /scratch/im21/[your_userid]/fastq_data/qc/
$ mv *.zip /scratch/im21/[your_userid]/fastq_data/qc/
$ mv *.html /scratch/im21/[your_userid]/fastq_data/qc/
Now we can navigate into this results directory and do a closer inspection of our output files.
$ cd /scratch/im21/[your_userid]/fastq_data/qc/
Running FastQC in Batch
In batch mode, instead of interactive mode, you must load the environment within the script you are trying to submit. This is why you have to perform the loading of environment, and module load..
#!/bin/bash
#PBS -l ncpus=2
#PBS -l mem=4GB
#PBS -l walltime=01:00:00
#PBS -l storage=gdata/im21
#PBS -P im21
#PBS -q normal
#PBS -l wd
#load the module
module load fastqc
#check the script run successfully
echo "check my script"
#cd into the locations
cd /scratch/im21/[your_userid]/fastq_data/
#fastqc
fastqc *.fastq*
Viewing the FastQC results
If we were working on our local computers, we could look at each of these HTML files by opening them in a web browser. However, to look at a summary version of the Fastqc html files- we need to create a summary file.
These files are currently on NCI Gadi, where our local computer cannot see them. And, since we are only logging into the NCI Gadi via the command line - it does not have any web browser setup to display these files either.
So the easiest way to look at these webpage summary reports will be to transfer them to our local computers (i.e. your laptop).
To transfer a file from a remote server to our own machines, we will use scp.
First we will make a new directory on our computer to store the HTML files we are transferring. Let’s put it on our desktop for now. Open a new tab in your terminal program (you can use the pull down menu at the top of your screen or the Cmd+t keyboard shortcut) and type:
$ mkdir -p ~/Desktop/FASTQC_HTML
Now we can transfer our HTML files to our local computer.
1) Check if you have a Mac/Linux or Windows operating system. 2) Open a new terminal/putty window where you are NOT logged into NCI Gadi.
For a Mac/Linux OS:
3) Navigate into a known location e.g. Desktop with the cd command.
For a Windows OS:
3) Navigate to a known location with the equivalent command as cd, which is pushd or popd
4) Using scp to move some information from scratch to your local computer.
$ scp [userID]@gadi.nci.org.au:"/scratch/im21/[your_userid]/fastq_data/qc/*.html" .
Understanding the Phred Quality Score
How the Phred quality score makes sense in terms of accuracy:
Note on using zsh
If you are using zsh instead of bash (macOS for example changed the default recently to zsh), it is likely that a
no matches founderror will be displayed. The reason for this is that the wildcard (“*”) is not correctly interpreted. To fix this problem the wildcard needs to be escaped with a “\”:
Now we can go to our new directory and open the 6 HTML files.
Depending on your system, you should be able to select and open them all at once via a right-click menu in your file browser.
Exercise
Could you discuss your results with your group or a neighbour? Which sample(s) look the best regarding per-base sequence quality? Which sample(s) look the worst?
Working with the FastQC text output
Our .zip files are compressed. They each contain multiple different types of output files for a single input FASTQ file. To view the contents of a .zip file, we can use the program unzip to decompress these files. Let’s try doing them all at once using a wildcard.
To unzip the files
$ unzip *.zip
This is the equivalent of running the for loop below.
$ for filename in *.zip
> do
> unzip $filename
> done
Let’s see what files are present within one of these output directories.
Use less to preview the summary.txt file for this sample.
$ cat SRR2589044_FASTQC/summary.txt
The summary file gives us a list of tests that FastQC ran, and tells us whether this sample passed, failed, or is borderline (WARN). Remember, to quit from less you must type q.
Other notes – optional
Quality encodings vary
Although we have used a particular quality encoding system to demonstrate the interpretation of read quality, different sequencing machines use different encoding systems. This means that, depending on which sequencer you use to generate your data, a
#may not indicate a poor quality base call.This mainly relates to older Solexa/Illumina data, but you must know which sequencing platform was used to generate your data, so that you can tell your quality control program which encoding to use. If you choose the wrong encoding, you run the risk of throwing away good reads or (even worse) not throwing away bad reads!
Same symbols, different meanings
Here we see
>as a shell prompt, whereas>is also used to redirect output. Similarly,$is used as a shell prompt, but, as we saw earlier, it is also used to ask the shell to get the value of a variable.If the shell prints
>or$, then it expects you to type something, and the symbol is a prompt.If you type
>or$yourself, it is an instruction from you that the shell should redirect output or get the value of a variable.
Key Points
Quality encodings vary across sequencing platforms.
forloops let you perform the same operations on multiple files with a single command.
Adapted from the Data Carpentry Intro to Command Line -shell genomics https://datacarpentry.org/shell-genomics/
Licensed under CC-BY 4.0 2018–2022 by The Carpentries
Licensed under CC-BY 4.0 2016–2018 by Data Carpentry